 |
Group leader։ Anush Kh. Khachatryan
PhD in Chemistry, Associate Professor |
| ORCID: 0000-0003-1210-9903 | |
| E-mail: khachatryan-ax@mail.ru | |
| Phone։ +37494884948 |
The Azomethine Group was formed in 1994 and transformed into a laboratory in 2012. During this period, the laboratory was headed by Doctor of Chemical Sciences Mushegh S. Sargsyan. In 2023, it became a group again. Since then, it has been led by Anush Kh. Khachatryan, who has a PhD in Chemistry and is an Associate Professor who continues the main area of research – studying the interaction between active electrophilic alkenes containing electron-accepting groups and active methylene compounds.
MAIN RESEARCH DIRECTION
Studies in Organic Synthesis
- Study of the interaction of imines, electrophilic alkenes (chalcones) and nucleophiles (C-H acids) containing an amide group,
- Study of the regioselectivity of the intramolecular cyclization of intermediates obtained as a result of the Michael reaction, including analysis of the possibilities of the Michael retro-reaction.
Synthesis of New Organic Systems
- Synthesis of multifunctional substituted carbo- and azacyclic compounds,
- Study of the biological properties of the obtained products.
GROUP STAFF
- A.Kh. Khachatryan ― Group leader, PhD in Chemistry., Associate Professor
- A.E. Badasyan ―Senior Researcher, PhD in Chemistry
- A.A. Sargsyan ― Researcher, PhD in Chemistry
- K.A. Avagyan ― Junior Researcher
- A.G. Simonyan ― Junior Researcher
- A.N. Zohrabyan ― Senior Laboratory Assistant
Five dissertations have been defended in the group.
PARTICIPATION IN GRANTS
- “RESEARCH SUPPORT PROGRAM FOR POSTGRADUATE STUDENTS AND YOUNG APPLICANTS – 2023” 23AA-1D001
- “RESEARCH ON THE EFFECTIVENESS OF PROMOTING THE GRANT PROGRAM -2025”: 25RG-1D082
BEST RESULTS
- The chemoselectivity of the reaction between araldimines and arylamides of acetoacetic acid has been revealed. It has been shown that intramolecular cyclization, depending on the electronic nature of the substituents present in the starting molecules, proceeds with the formation of either 3-aryl-N,6-diaryl-3-hydroxy-1,3-dimethyl-5-oxo-2-oxa-6-azabicyclo[2,2,2]octane-7-carboxamides (in the heterocycle) or 4-hydroxy-4-methyl-6-oxo-2-aryl-N1,N3-diarylcyclohexane-1,3-dicarboxamides (in the carbocycle), or a mixture thereof.
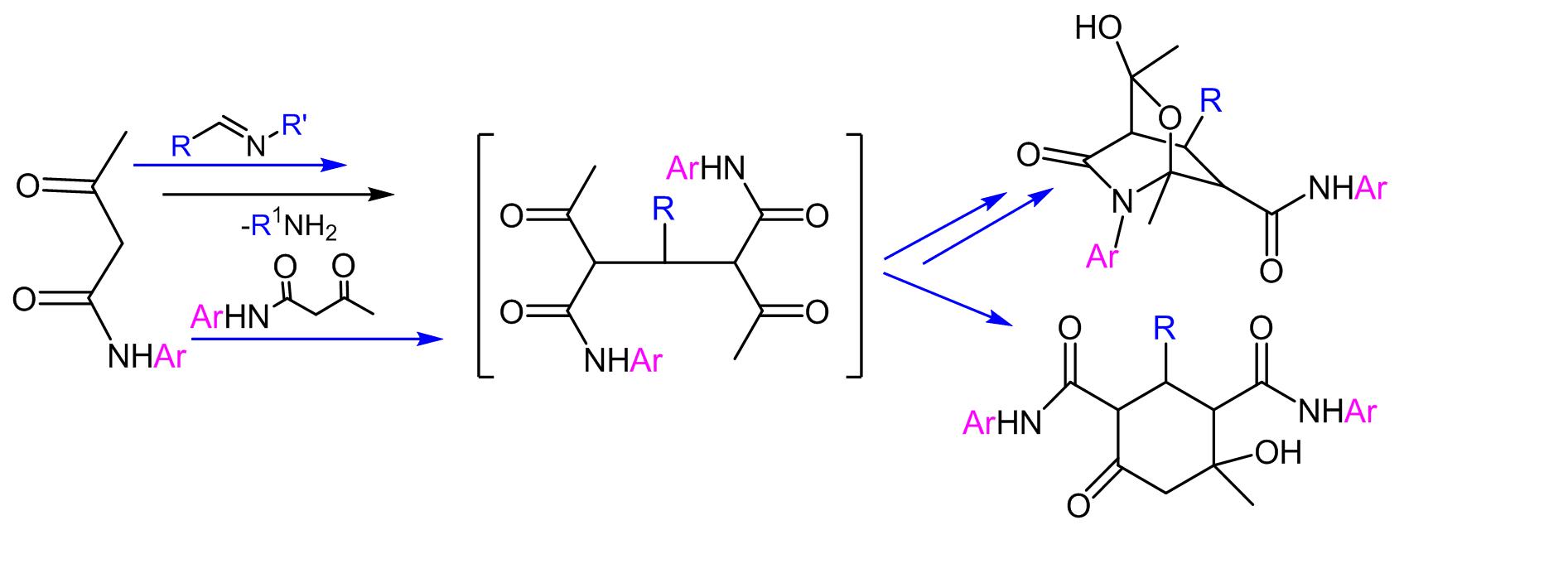
Chem. J. Armenia, 2015, v. 68, № 2, pp. 266-275
- It has been shown that the reaction of ethyl 6-aryl-5-(arylcarbamoyl)-2-hydroxy-2-methyl-4-oxocyclohexanecarboxylates with hydroxylamine hydrochloride, in addition to the expected corresponding oximes, in some cases also leads to the formation of ethyl 4-hydroxy-4-methyl-6-oxo-2-arylpiperidine-3-carboxylates. A separate experiment confirmed that the latter are not formed from the starting cyclohexanes, but are the result of the subsequent reaction of the corresponding oximes with hydroxylamine hydrochloride.
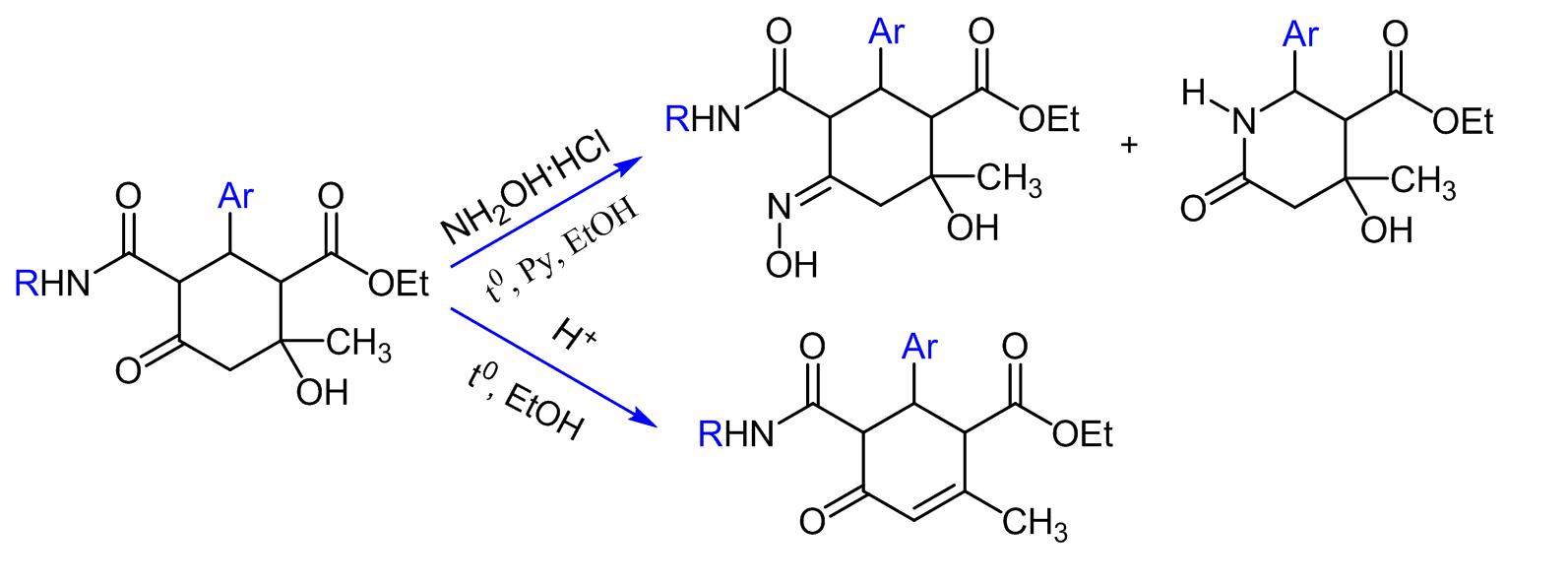
Chem. J. Armenia, 2019, v. 72, № 3, pp. 282-291
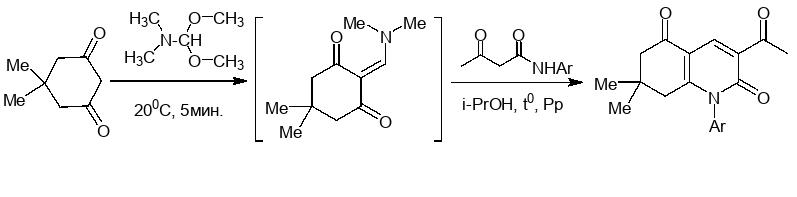
Chem. J. Armenia, 2021, v. 74, № 1-2, pp. 113-121
- A method for obtaining diethyl 6-amino-1,4-diaryl-2-oxo-1,2,3,4-tetrahydropyridine-3,5-dicarboxylates from the interaction of arylmethylidenedianacetic acid esters and arylamidoesters of malonic acid has been developed.
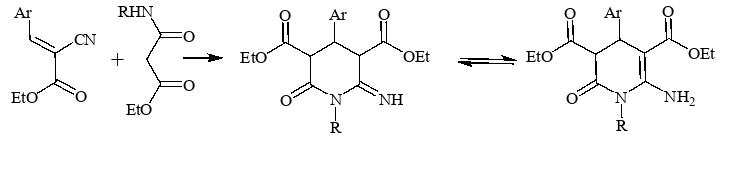
Russ. J. Gen. Chem., 2023, V. 93, № 4, pp. 787–794
https://doi.org/10.1134/S1070363223040035
- Diethyl 6-amino-1-aryl-2-oxo-1,2-dihydropyridine-3,5-carboxylates were synthesize
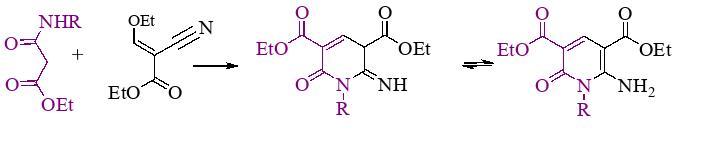
Russ. J. Gen. Chem., 2024, V. 94, № 4, pp. 462–468.
https://doi.org/10.31857/S0044460X24040011
- A new accessible method for the synthesis of ethyl 2-amino-1-(aryl)-5-(arylcarbamoyl)-6-oxo-1,6-dihydropyridine-3-carboxylates has been developed.
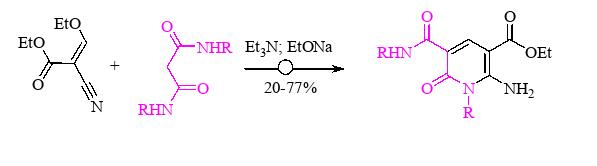
Chem․ Heterocycl. Comp․, 2024, 60(3/4), 150–155.
https://doi.org/10.1007/s10593-024-03311-5
LIST OF THE BEST PUBLICATIONS OF RECENT YEARS
- S. Hayotsyan,A. A. Sargsyan, S. G. Kon’kova, A. Kh. Khachatryan, A. E. Badasyan, K. A. Avagyan, M. S. Sargsyan Russ. J. Org. Chem. 2019. Vol. 55. № 2. P. 282–286.
- S. Hayotsyan, A. A. Sargsyan, S. G. Kon’kova, A. Kh. Khachatryan, A. E. Badasyan, K. A. Avagyan, H. A. Panosyan, A. G. Ayvazyan, M. S. Sargsyan Russ. J. Org. Chem.. 2019. Vol. 55. № 4. P. 469-472.
- Sargsyan М. С., Avagyan К. А., Sargsyan А. А., Badasyan E., Khachatryan A. Kh., Ayvazyan A. G.; Balyan А. А., Kon’kova S. G., Ayotsyan S. S. Chem. J. Armenia 2019, 72(3), 304-313.
- А. А. Саргсян, С. С. Aйоцян, А. Х. Хачатрян, А. Э. Бадасян, Г. А. Паносян, К. А. Авагян, С. Г. Конькова и М. С. Саргсян Хим. ж. Армении, 2019. Т.72. № 3. С. 282-291.
- S. Sargsyan, K. A. Avagyan, A. A. Sargsyan, A. E. Badasyan, A. Кh. Khachatryan, S. G. Konkova, A. G. Manukyan, G. M. Makaryan and S. S. Hayotsyan Chem. J. Armenia. 2019. Vol.72. № 4. P.502-508.
- М. С. Саргсян, К. А. Авагян, А. А. Саргсян, А. Э. Бадасян, А. Х. Хачатрян, А. Г. Манукян, А.Г. Айвазян Хим. ж. Армении. 2020. Т.73. № 1. С. 61-68.
- А. А. Саргсян, А. Х. Хачатрян, А. Э. Бадасян, К. А. Авагян, А. Г. Манукян , Г.А. Паносян, А.Г. Айвазян, М. С. Саргсян Хим. ж. Армении. 2021. Т.74. №1-2. С.113-121.
- Sargsyan А. А., Khachatryan А. Kh., Badasyan А. E., Avagyan К. А., Manukyan А. G., Paposyan G. А., Sargsyan М. S. Chem. Armenia 2022, 75, № 1, 54-61.
- A. Avagyan, M. S. Sargsyan, A. E. Badasyan, A. A. Sargsyan, A. G. Manukyan, G. A. Panosyan, A. G. Ayvazyan, A. Kh. Khachatryan Russ. J. Gen. Chem. 2023, V. 93, № 4, pp. 787–794. https://doi.org/10.1134/S1070363223040035
- A. Khachatryan, K. A. Avagyan, A. A. Sargsyan, A. E. Badasyan Russ. J. Org. Chem., 2024, Vol. 60, No. 4, pp. 743–746. 10.1134/S1070428024040250
- Anush Kh. Khachatryan, Katya A. Avagyan, Anush А. Sargsyan, Anait G. Simonyan, Henrik А. Panosyan, Armen G. Ayvazyan, Alik E. Badasyan Chem․ Comp․ 2024, 60(3/4), 150–155 https://hgs.osi.10.1007/s10593-024-03311-5 https://hgs.osi.lv/index.php/hgs/article/view/8679
- А.Х. Хачатрян, К.А. Авагян, А.Э. Бадасян ЖОХ, 2024, т. 94, № 4, с. 462–468. https://doi.org/10.31857/S0044460X24040011ECIZJX
- A. Avagyan, A.E. Badasyan, A.A. Sargsyan, A.Kh. Khachatryan «New Emerging Trends in Chemistry» Conference (NewTrendsChem-2023), September 24-28, 2023, p.114. Yerevan, Armenia.
- A. Avagyan, A.E. Badasyan, A.Kh. Khachatryan VI All-Russian Conference on Organic Chemistry, September 23-27, 2024, p.165, Institute of Organic Chemistry RAS, Moscow.
- Kh. Khachatryan, A.A. Sargsyan, K.A. Avagyan, A.G. Simonyan, A.E. Badasyan, V.V. Dotsenko «New Emerging Trends in Chemistry» Conference (NewTrendsChem-2025), September 21-25, Book of Abstracts, 2025, p.199. Yerevan, Armenia.
- Kh. Khachatryan., K.A. Avagyan «New Emerging Trends in Chemistry» Conference (NewTrendsChem-2025), Book of Abstracts, September 21-25, 2025, p.151. Yerevan, Armenia.