Design and synthesis of compounds, regulating the activity of the cardiovascular system

Head of the Laboratory
Aghekyan Asya, Candidate of Chemical Sciences
ORСID: ID 0000-0001-6151-4951
Telephone: (+37410) 285–101
E-mail: aaghekyan@mail.ru
Research Directions
The laboratory focuses on the synthesis of novel compounds with antihypoxic, antiarrhithmic, antioxidant, antimonoaminoxidase, anticonvulsant and antimicrobial activity in the series of tetrahydroisoquinoline, 1,3-benzodioxole, 1,4-benzodioxane and five-membered heterocycles with two or three heteroatoms.
The main direction of the laboratory’s research is the synthesis of new oxygen- and nitrogen-containing heterocyclic compounds that regulate the activity of the cardiovascular and central nervous systems, as well as possessing antimicrobial, antihypoxic, antimonoaminoxidase, anticonvulsant and antioxidant activity, among 1,4-benzodioxane, 4-spiro-substituted tetrahydroisoquinoline and its non-cyclized analogues.
History of the Laboratory. Founded and headed by Varduhi Afrikyan, Candidate of Chemical Sciences, in 1955. The laboratory was engaged in the synthesis of new aromatic and heterocyclic amino ethers affecting the cardiovascular system and the study of their biological properties, with the aim of revealing the relationship between chemical structure and biological activity. As a result of biological research, a number of drugs were discovered: Fubromegan, Quateron and Gangleron, which have found wide application in medical practice.
In 1970, the laboratory was headed by Doctor of Chemical Sciences, Professor Eduard Margaryan, who, expanding the scope of the research, began work in the field of synthesis of diarylpropionic acids, arylalkylamines, condensed heterocyclic systems: indole, isoquinoline, benzazepine, benzodioxane, isochroman derivatives. As a result, a number of potential drugs were discovered: Fobufol, Difalkin, Emacor, Beditin, Mesedin.
Since 2011, the laboratory has been headed by Asya Aghekyan, Candidate of Chemical Sciences, who has continued the main direction of research – the targeted synthesis of biologically active compounds from nitrogen- and oxygen-containing heterocyclic annelated systems. The results of this work have been presented in numerous scientific publications.
Current activities
- The laboratory employs 8 highly qualified specialists, including 4 PhD
Best Results
✔ Optimal conditions were developed for the synthesis of 4-spirocycloalkane-substituted tetrahydroisoquinoline derivatives containing at the second position amino- and sulfanylamide, alkanol and heterylalkyl pharmacophore fragments
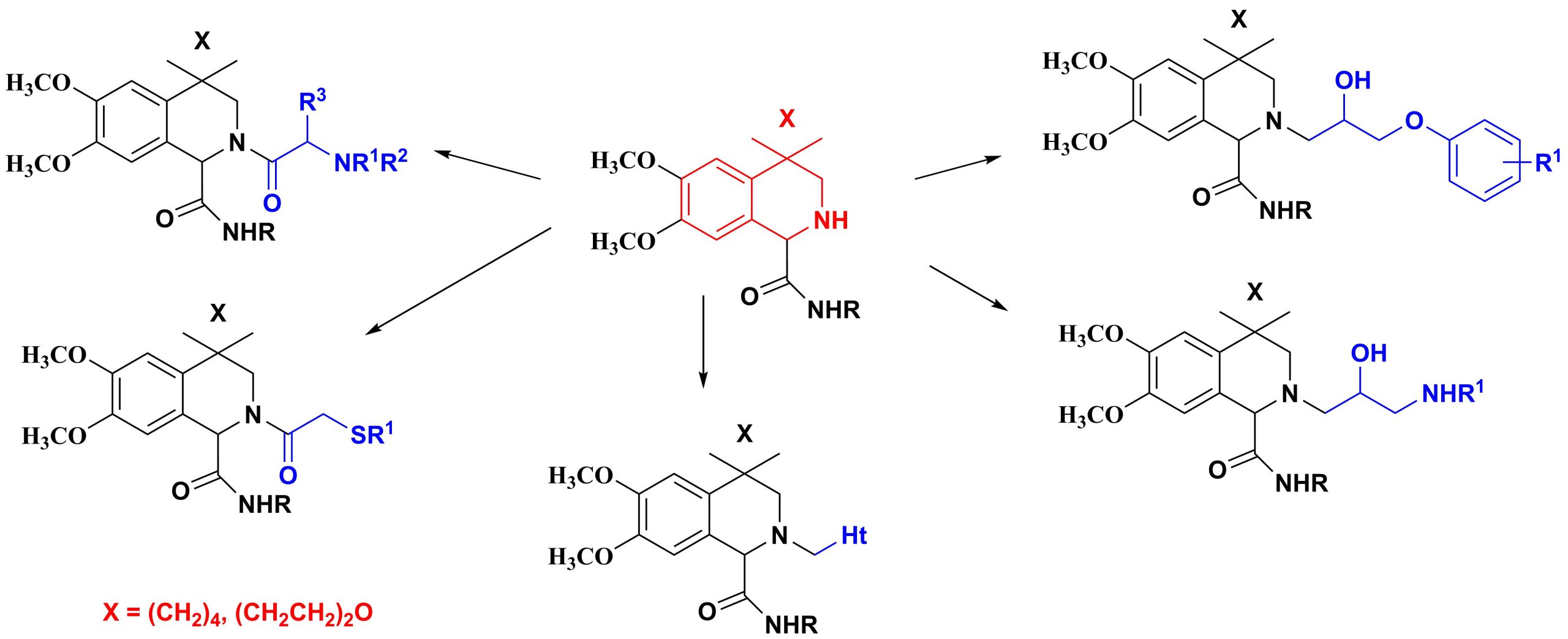
Russ. J. Org. Chem., 2013, v. 49, No. 11, pp. 1632-1636. doi: 10.1134/S1070428013110122
Russ. J. Org. Chem., 2016, v. 52, No. 5, pp. 689-693. doi:10.1134/S1070428016050122
Russ. J. Org. Chem., 2022, v. 58, No. 11, pp. 1581-1588. doi:10.1134/S1070428022110045
Russ. J. Org. Chem., 2019, v. 55, No. 3, pp. 302-307. doi:10.1134/S1070428019030047
Russ. J. Org. Chem., 2015, v. 51, No. 2, pp. 221-225. doi:10.1134/S1070428015020153
✔ A new approach for the synthesis of tetrahydroisoquinoline derivatives containing two methyl groups at the fourth position based on substituted benzylamines has been proposed.
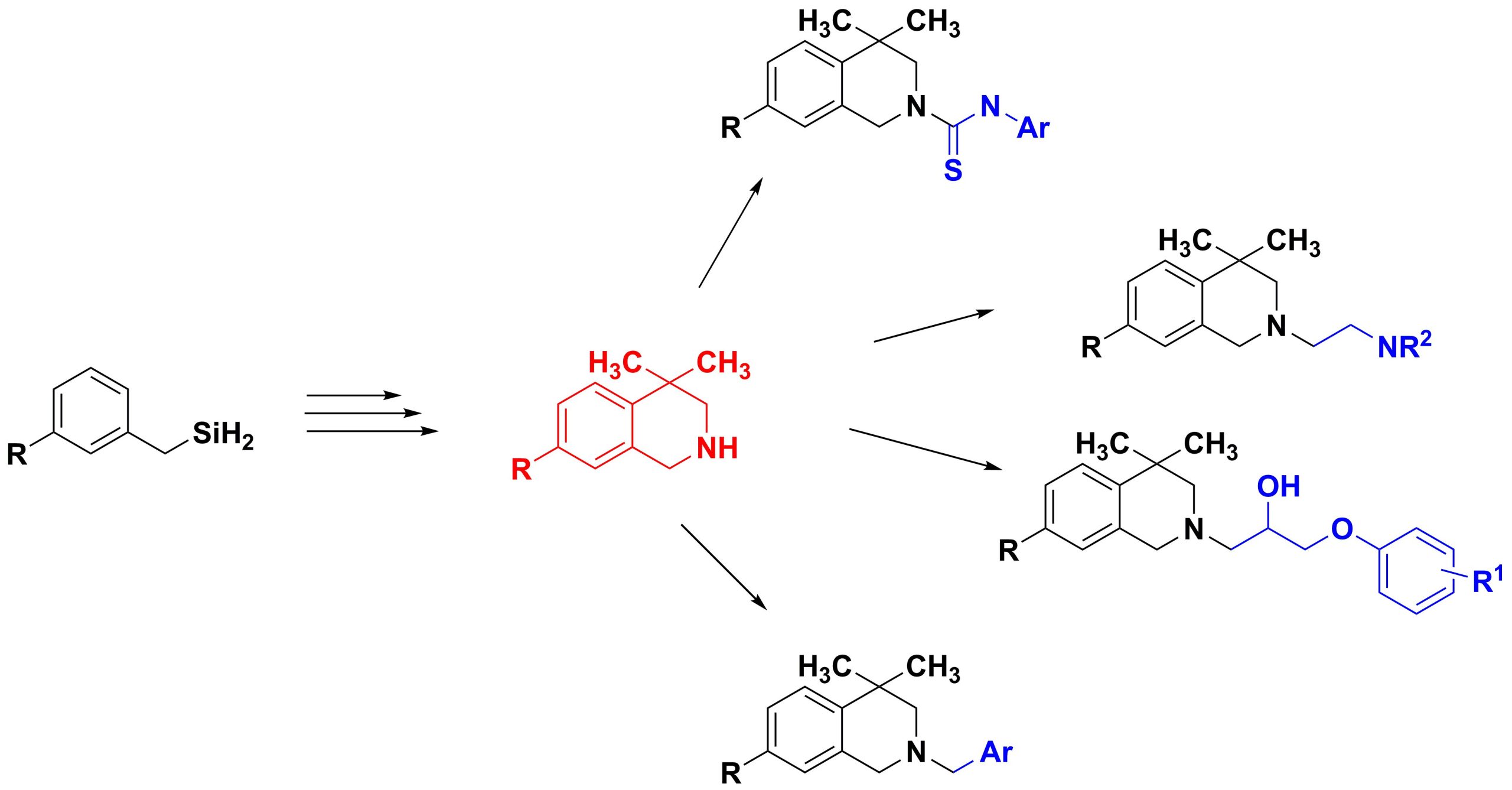
Russ. J. Org. Chem., 2017, v. 53, No. 3, pp. 362-365. doi:10.1134/S1070428017030083
Russ. J. Org. Chem., 2022, v. 58, No. 10, pp. 1409-1415. doi:10.1134/S1070428022100049
✔ Methods for the synthesis of various derivatives of 1,4-benzodioxane containing at the second position a substituted 1,2,4-triazole ring have been developed.
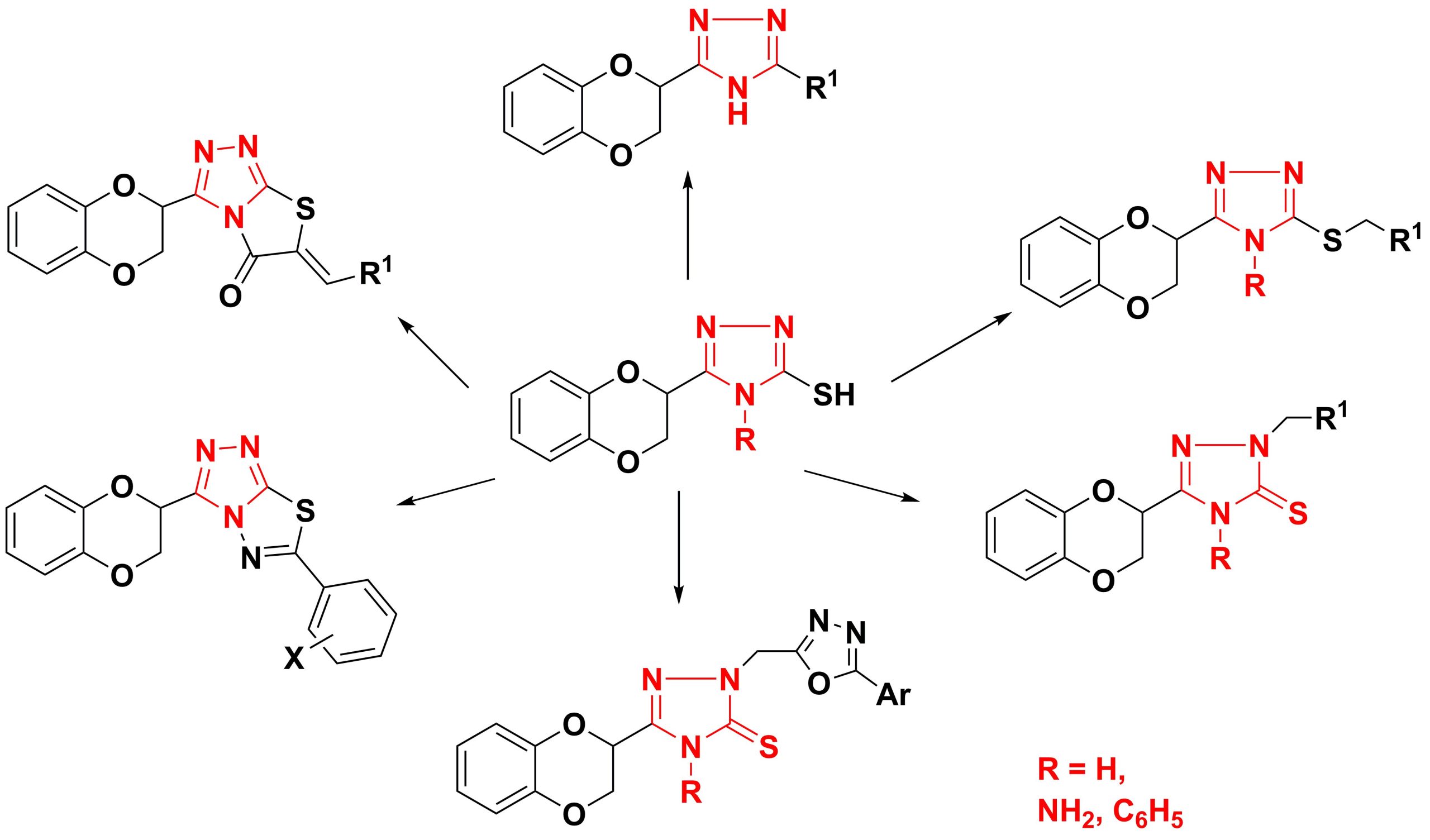
Russ. J. Org. Chem., 2017, v. 53, No. 3, pp. 428-432. doi:10.1134/S1070428017030198
Russ. J. Gen. Chem., 2018, v. 88, No. 4, pp. 839-842. doi:10.1134/S1070363218040345
Russ. J. Org. Chem., 2020, v. 56, No. 3, pp. 436-439. doi:10.1134/S1070428020030112
Chem. J. Armenia, 2023, v. 76 № 1-2, pp. 104-108
Russ. J. Org. Chem., 2021, v. 57, No. 7, pp. 1068-1072. doi:10.1134/S107042802107006X
✔ Synthesis and certain transformations of 1,4-benzodioxane and their substituted derivatives containing 1,3,4-oxadiazole and 1,3,4-thiadiazole heterocyclic rings were carried out.
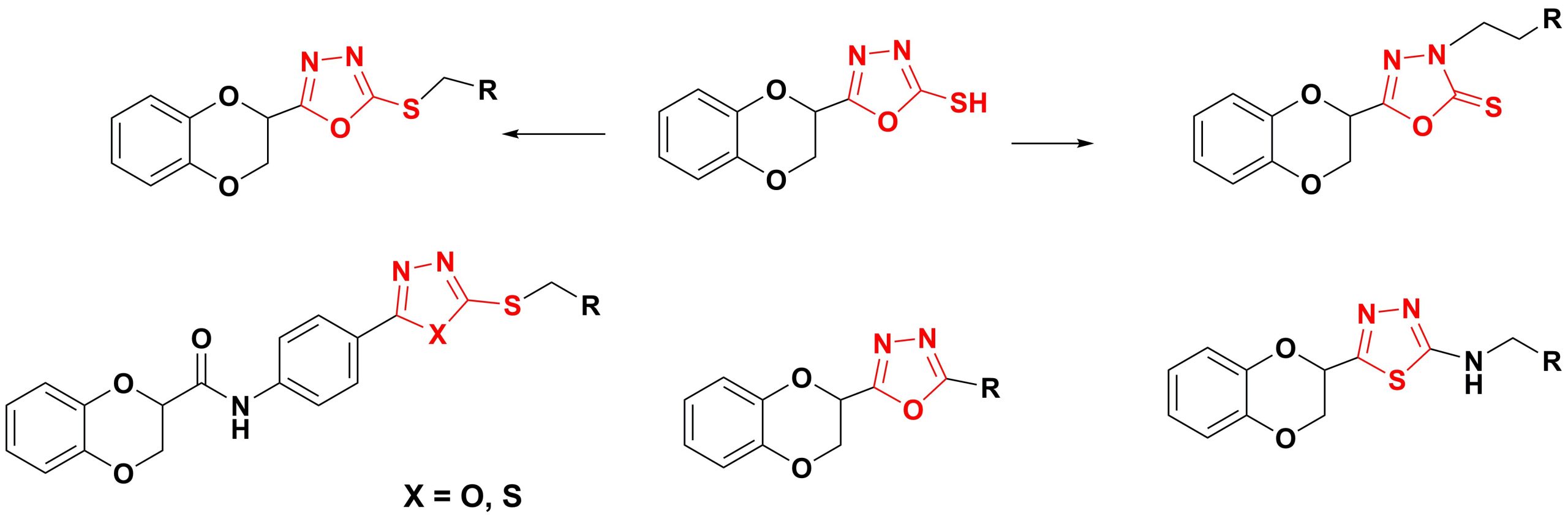
Russ. J. Org. Chem., 2020, v. 56, No. 3, pp. 385-389. doi:10.1134/S1070428020030033
Russ. J. Org. Chem., 2014, v. 50, No. 3, pp. 434-438. doi:10.1134/S1070428014030233
Russ. J. Org. Chem., 2024, v. 60, No. 9, pp. 1685-1691. doi:10.1134/S1070428024090100
Russ. J. Org. Chem., 2017, v. 53, No. 12, pp. 1905-1908. doi:10.1134/S1070428017120259
✔ The synthesis of diamide derivatives of p-aminobenzoic acid containing an arylcycloalkane and 1,4-benzodioxane fragments was developed and implemented.
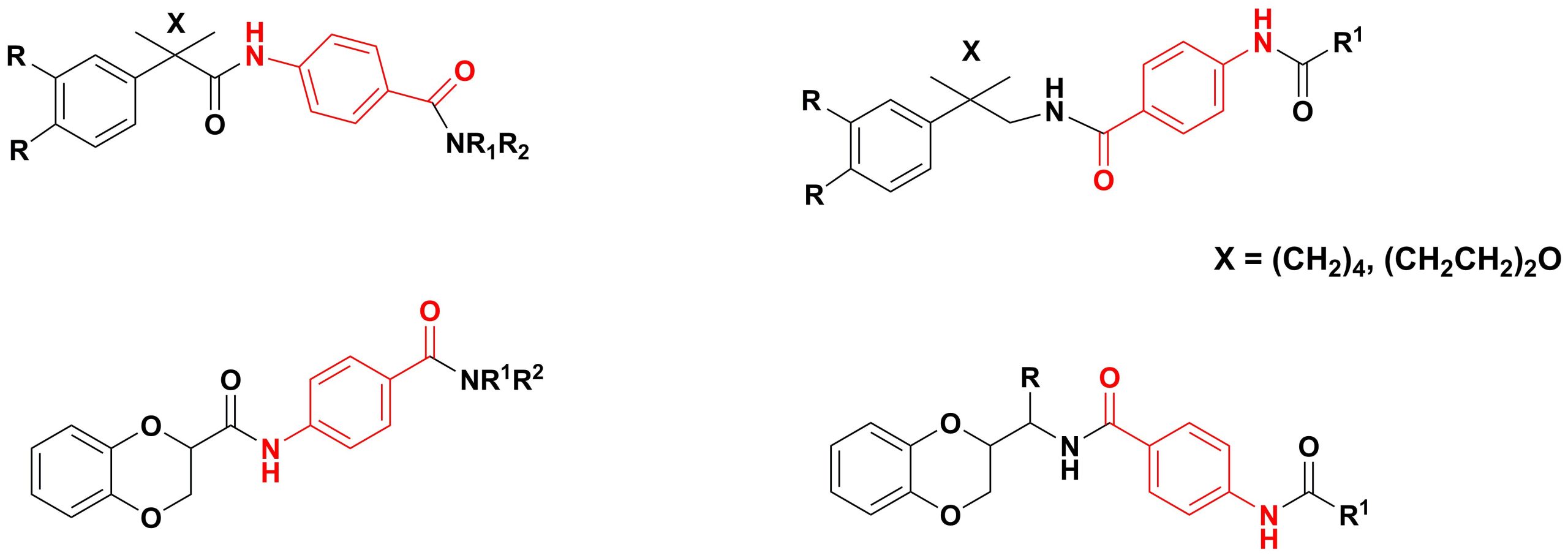
Russ. J. Gen. Chem., 2015, v. 85, No. 5, pp. 1057-1062. doi:10.1134/S1070363215050096
Chem. J. Armenia, 2012, v. 65, № 2, pp. 230-238
Chem. J. Armenia, 2016, v. 69, № 1-2, pp. 111-120
Russ. J. Org. Chem., 2012, v. 48, No. 7, pp. 972-976. doi:10.1134/S1070428012070147
✔ The synthesis of new aryloxypropanolamines based on arylcyclopentane(tetrahydropyran, dimethyl)methylamines was developed and implemented
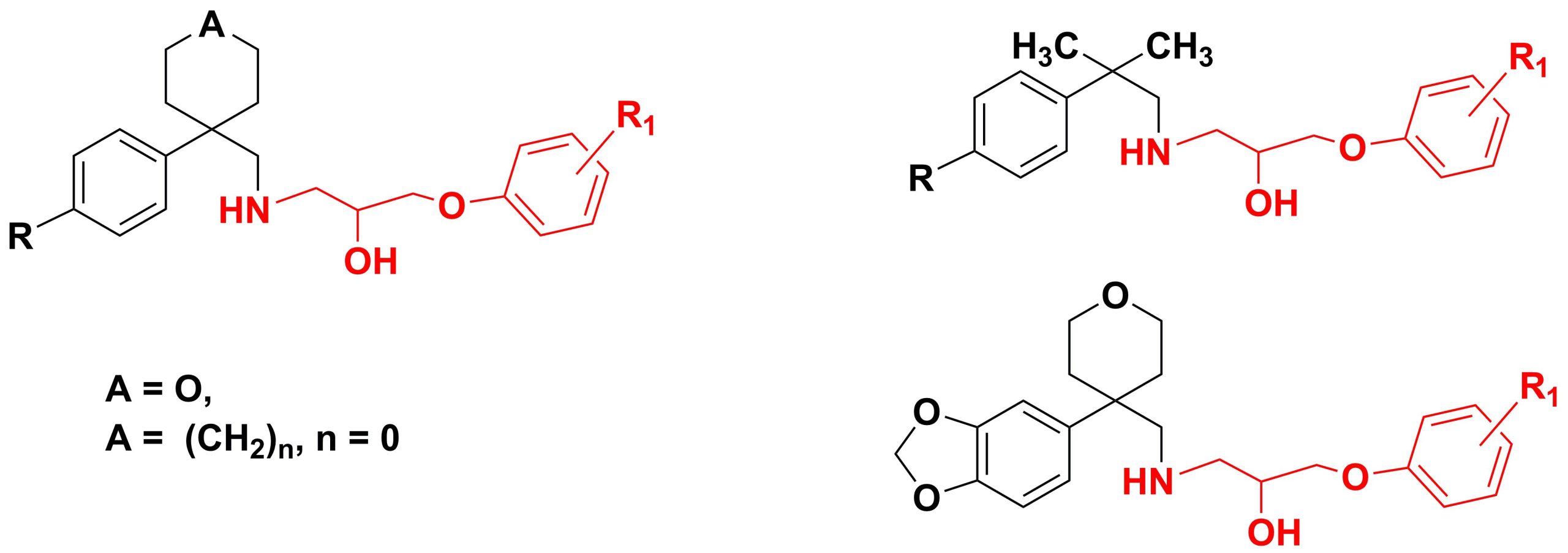
Russ. J. Org. Chem., 2016, v. 52, No. 2, pp. 209-213. doi:10.1134/S1070428016020081
Russ. J. Org. Chem., 2023, v. 59, No. 5, pp. 756-763. doi:10.1134/S1070428023050020
Russ. J. Org. Chem., 2024, v. 60, No. 1, pp. 18-24. doi:10.1134/S1070428024010032
✔ Various routes have been developed to synthesize different diamide derivatives of dibasic carboxylic acids (oxalic, succinic, and maleic).
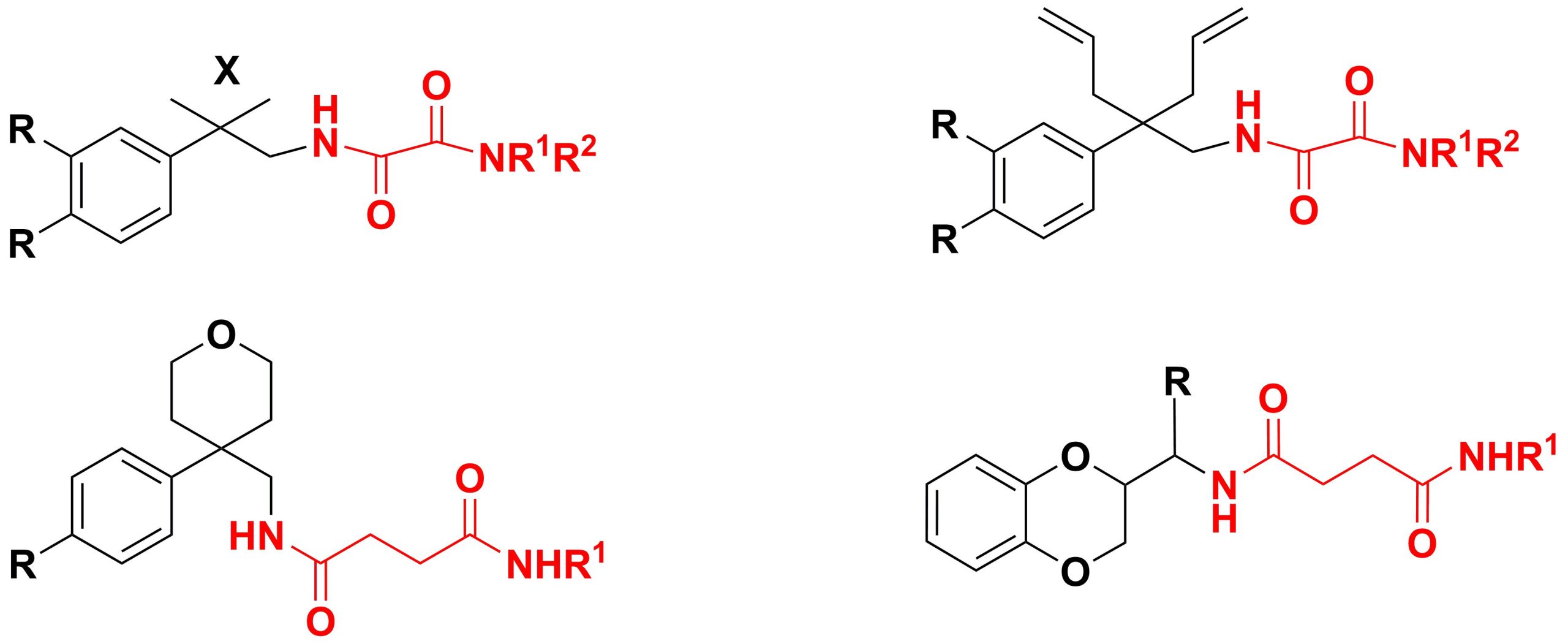
Russ. J. Org. Chem., 2013, v. 49, No. 7, pp. 1083-1086. doi:10.1134/S1070428013070221
Chem. J. Armenia, 2012, v. 65, № 2, pp. 215-223
Chem. J. Armenia, 2012, v. 65, № 3, pp. 332-341
Chem. J. Armenia, 2013, v. 66, № 4, pp. 628-635
Chem. J. Armenia, 2012, v. 65, № 1, pp. 111-117
Russ. J. Org. Chem., 2018, v. 54, No. 6, pp. 886-891. doi:10.1134/S1070428018060106
Russ. J. Org. Chem., 2024, v. 60, No. 11, pp. 2117-2124. doi:10.1134/S1070428024110046
✔ New aryl-, 1,3-benzodioxole- and 1,4-benzodioxanylcycloalkane(tetrahydropyran)carboxylic acid aminoamide and aminoester derivatives have been developed and synthesized for the first time.
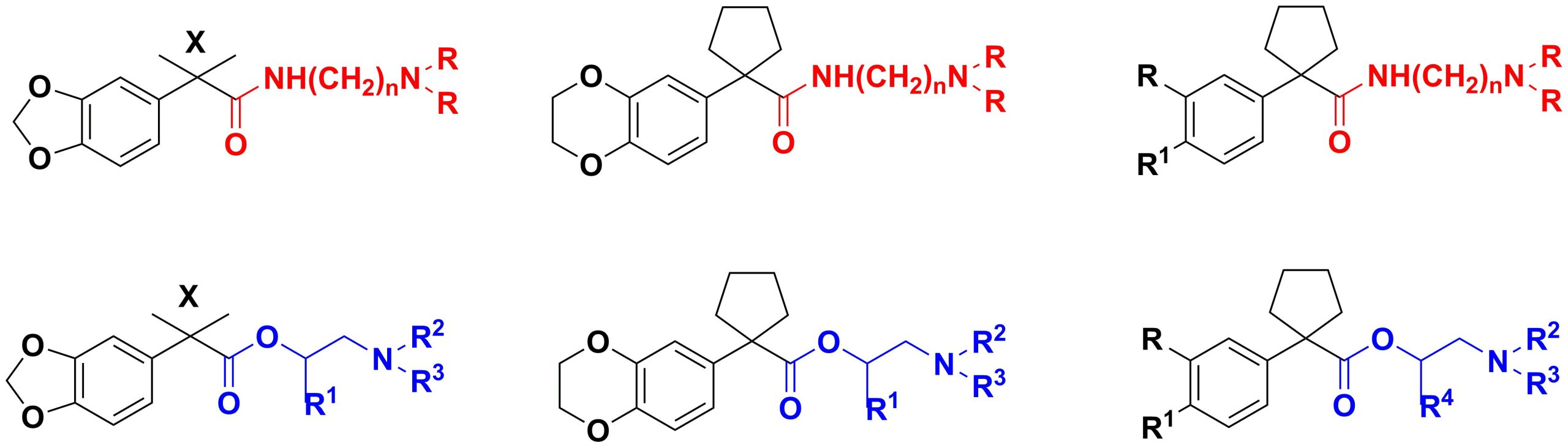
Russ. J. Gen. Chem., 2019, v. 89, No. 5, pp. 1051-1054. doi:10.1134/S107036321905027X
Russ. J. Org. Chem., 2024, v. 60, No. 6, pp. 1028-1035. doi:10.1134/S1070428024060071
Russ. J. Org. Chem., 2019, v. 55, No. 6, pp. 796-799. doi:10.1134/S1070428019060095
Russ. J. Org. Chem., 2025, v. 61, No. 3, pp. 403-411. doi:10.1134/S107042802560010X
✔ Methods for obtaining compounds with oxygen-containing heterocycles (1,4-benzodioxane, tetrahydropyran, furan) at the second position of the 1,3,4-oxadiazole ring have been developed.
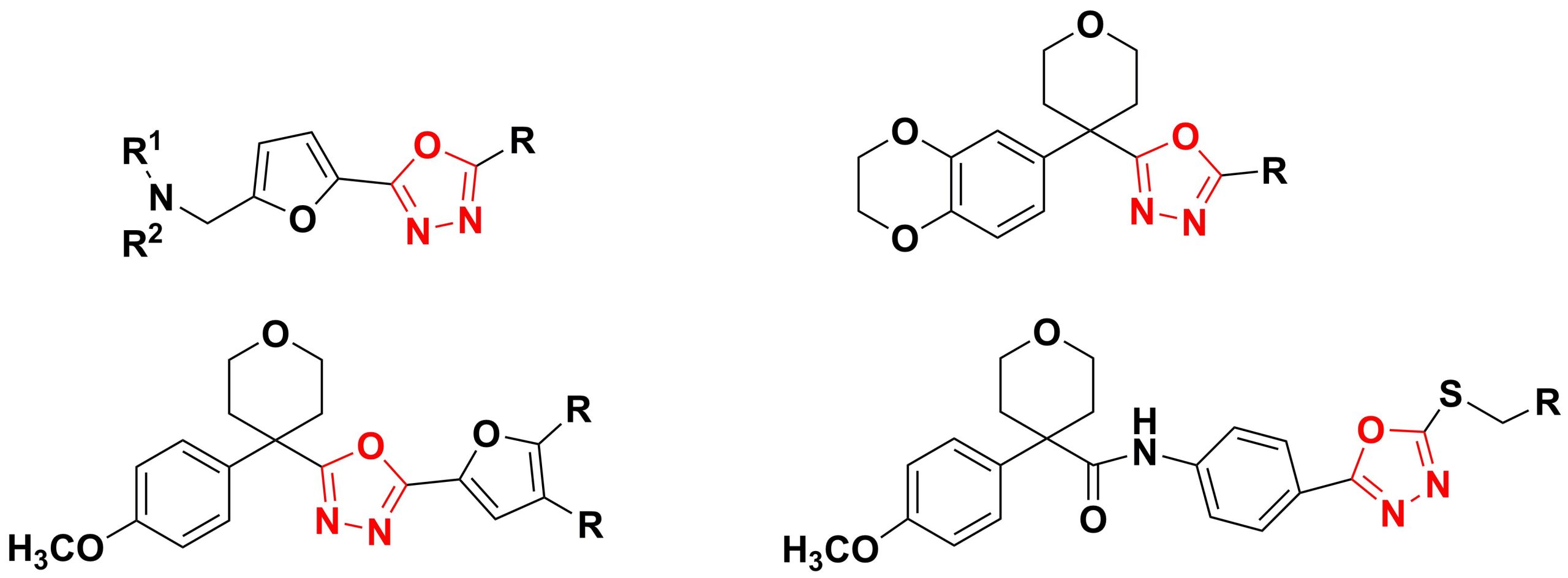
Russ. J. Org. Chem., 2025, v. 61, No. 1, pp. 73-79. doi:10.1134/S1070428024602966
Russ. J. Org. Chem., 2024, v. 60, No. 4, pp. 655-663. doi:10.1134/S1070428024040146
Russ. J. Org. Chem., 2020, v. 56, No. 2, pp. 281-286. doi:10.1134/S1070428020020177
✔ Methods for the synthesis of thienopyrimidines containing fragments of 1,4-benzodioxane and phenylcyclohexanecarbonitrile have been proposed, which were carried out via the cyclization reaction of 2-amino-3-carbethoxy-substituted thiophenes.
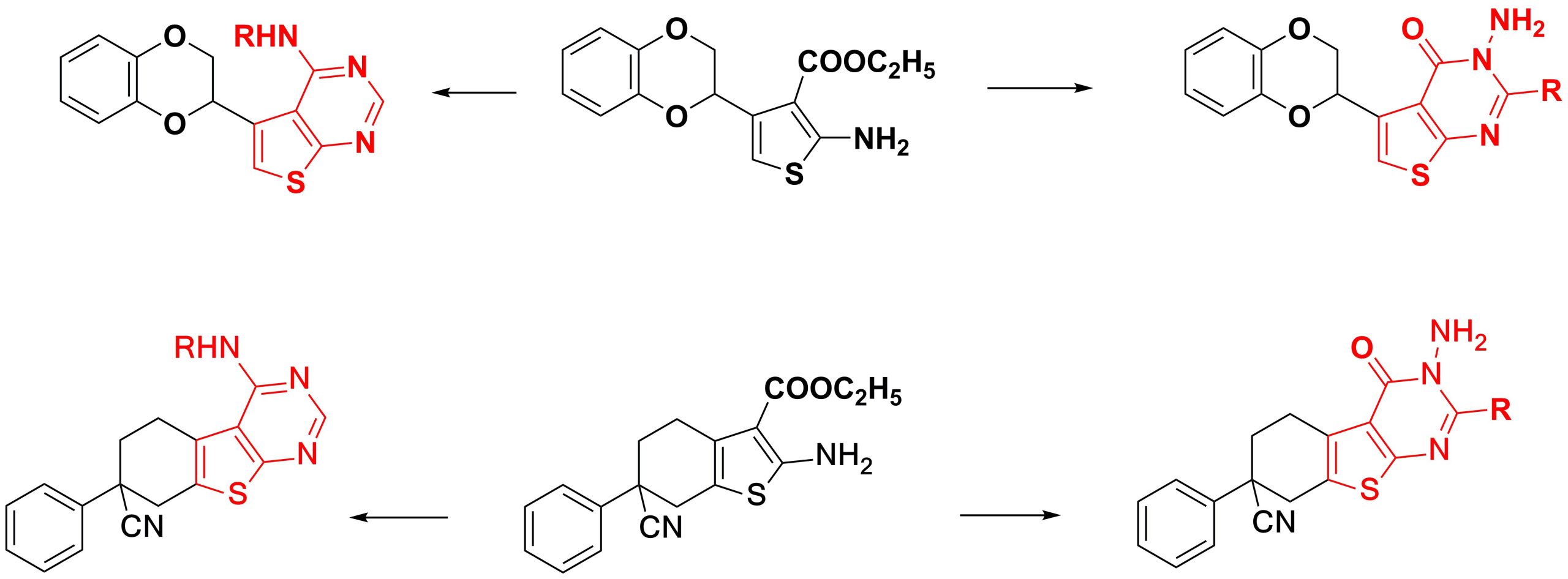
Russ. J. Org. Chem., 2019, v. 55, No. 5, pp. 598-601. doi:10.1134/S1070428019050038
Russ. J. Org. Chem., 2022, v. 58, No. 7, pp. 977-981. doi:10.1134/S1070428022070053
Russ. J. Org. Chem., 2024, v. 60, No. 3, pp. 397-402. doi:10.1134/S1070428024030047
Russ. J. Org. Chem., 2020, v. 56, No. 3, pp. 440-445. doi:10.1134/S1070428020030124
Russ. J. Org. Chem., 2021, v. 57, No. 10, pp. 1638-1642. doi:10.1134/S1070428021100110
✔ The antihypoxic, antimonoaminoxidase, antioxidant, antiarrhythmic, anticonvulsant, and antibacterial properties of the synthesized compounds have been investigated.
The best works of recent years
Russ. J. Org. Chem., 2021, v. 57, No. 2, pp. 195-202. doi:10.1134/S1070428021020093
Russ. J. Org. Chem., 2021, v. 57, No. 7, pp. 1068-1072. doi:10.1134/S107042802107006X
Russ. J. Org. Chem., 2021, v. 57, No. 10, pp. 1638-1642. doi:10.1134/S1070428021100110
Russ. J. Org. Chem., 2022, v. 58, No. 7, pp. 977-981. doi:10.1134/S1070428022070053
Russ. J. Org. Chem., 2022, v. 58, No. 10, pp. 1409-1415. doi:10.1134/S1070428022100049
Russ. J. Org. Chem., 2022, v. 58, No. 11, pp. 1581-1588. doi:10.1134/S1070428022110045
Russ. J. Org. Chem., 2023, v. 59, No. 11, pp. 1884-1891. doi:10.1134/S1070428023110064
Russ. J. Org. Chem., 2024, v. 60, No. 1, pp. 18-24. doi:10.1134/S1070428024010032
Russ. J. Org. Chem., 2024, v. 60, No. 3, pp. 397-402. doi:10.1134/S1070428024030047
Russ. J. Org. Chem., 2024, v. 60, No. 9, pp. 1685-1691. doi:10.1134/S1070428024090100
Russ. J. Org. Chem., 2024, v. 60, No. 4, pp. 655-663. doi:10.1134/S1070428024040146
Russ. J. Org. Chem., 2024, v. 60, No. 11, pp. 2117-2124. doi:10.1134/S1070428024110046
Russ. J. Org. Chem., 2025, v. 61, No. 1, pp. 73-79. doi:10.1134/S1070428024602966
Russ. J. Org. Chem., 2025, v. 61, No. 3, pp. 403-411. doi:10.1134/S107042802560010X