Head of the laboratory

Sahak P. Gasparyan , Doctor of Chemical Sciences
ORСID: 0000-0003-2341-724X
Tel.: (+37410) 284-033
Email: g_sahak@yahoo.com
The main direction of the laboratory’s research − the synthesis of new compounds with
antibacterial, antiviral and antitumor activity in the series of pyrrolidines, pyrimidines and
azadamantanes
History of the Antibiotic Synthesis Laboratory
It was founded and headed in 1970 by PhD in Chemistry Shushanik Levon Mndzhoyan. The
laboratory was engaged in the synthesis of new semi-synthetic (s/s) penicillins and cephalosporins
and the study of their antibacterial and antitumor properties in order to identify the relationship
between chemical structure and biological activity. As a result of biological research, a number of
active compounds were discovered, among which one can single out the semi-synthetic penicillin
“Fecillin” (sodium salt of 1-phenylcyclopentyl-1-carboxamidopenicillic acid), which has low
toxicity and high antibacterial activity.
Since 1997, the laboratory has been headed by PhD Ashot Hovhanes Martirosyan, who, having
expanded the scope of research, began to synthesize new derivatives of cyclic α-amino acids, in
particular, prolines and azetidines.
Since 2009, the laboratory has been headed by Doctor of Chem. Sci. Saak Paruyr Gasparyan,
who continued the main direction of research − the synthesis of effective biologically active
compounds in the series of pyrrolidines, pyrimidines and azaadamantanes. Many scientific papers
have been published and patents have been received both for the synthesis of these compounds and
for the demonstration of their biological properties.
Current activities
The laboratory employs 8 highly qualified specialists and 1 student, including:
- 1 doctor of science
- 3 PhD
Best results
✔️ Affordable methods have been developed and proposed, and a series of 2-arylpyrrolidine derivatives have been synthesized using them.
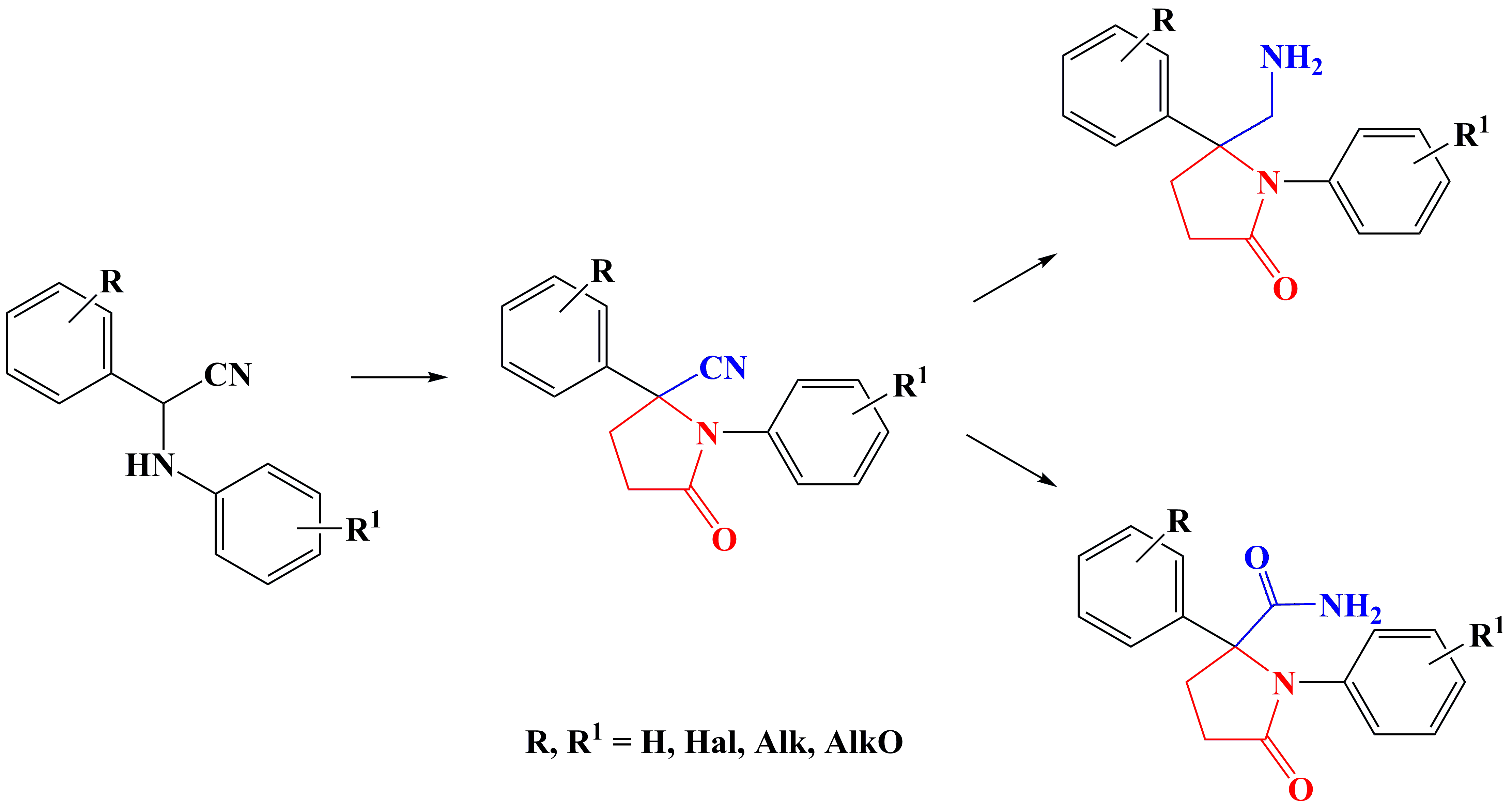
Pharm. Chem. J., 2012, v. 46, № 6, pp. 331-333. doi: 10.1007/s11094-012-0792-2
Chem. J. Armenia, 2014, v. 67, № 2-3, pp. 239-246
Chem. J. Armenia, 2016, v. 69, № 3, pp. 333-340
Med. Chem. Res., 2017, v. 26, № 1, pp. 101-108. doi: 10.1007/s00044-016-1731-7
✔️Phenyl-substituted hexahydropyrrolopyrazine-1,4- and perhydropyrrolodiazepine-1,5-diones were developed and synthesized for the first time
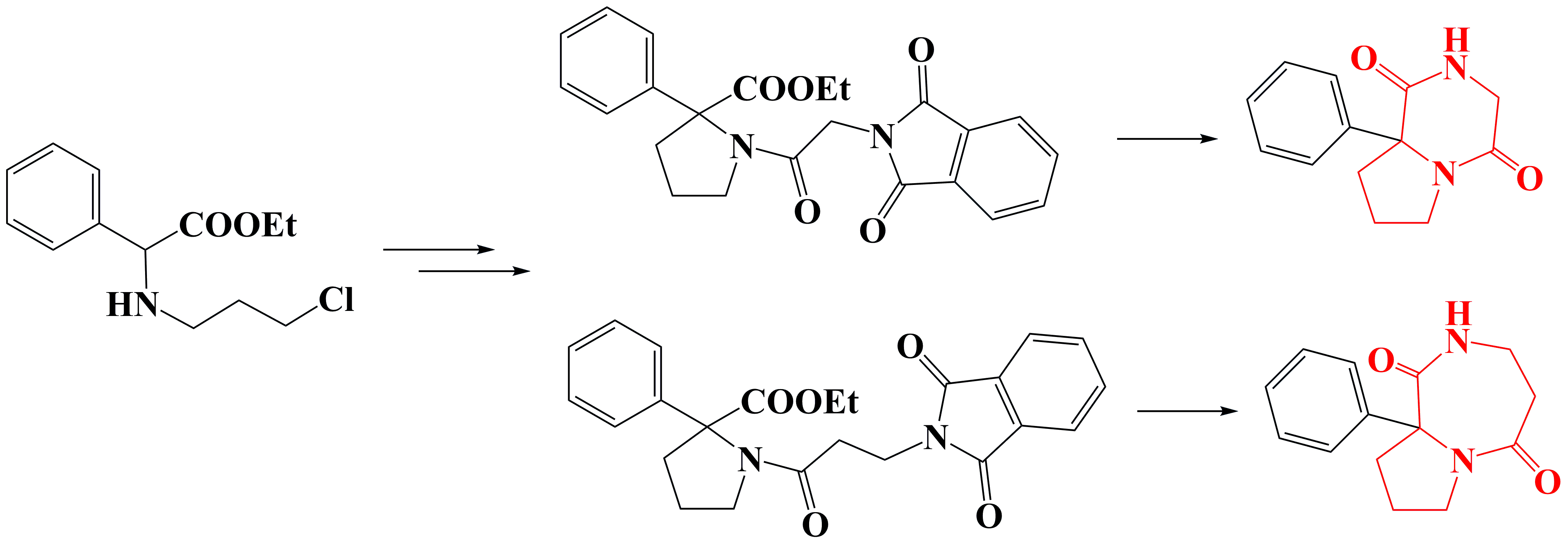
Chem. J. Armenia, 2014, v. 67, № 2-3, pp. 315-320
✔️The synthesis of new pyrimidine derivatives of C-azanucleosides has been developed and
implemented
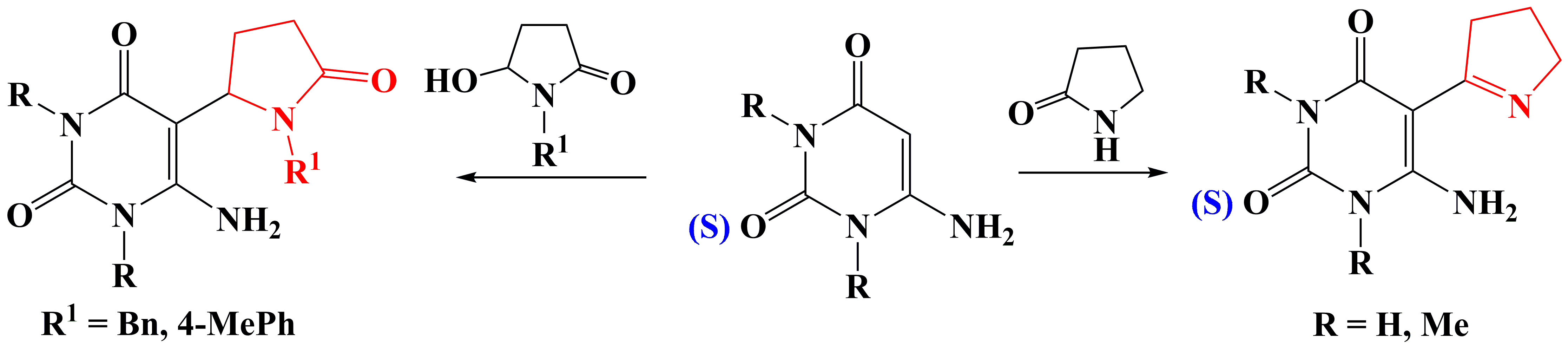
Tetr. Lett., 2012, v. 51, № 2, pp. 231-233. doi: 10.1016/j.tetlet.2009.11.010
Russ. J. Org. Chem., 2016, v. 52, № 11, pp. 1646-1653. doi: 10.1134/S1070428016110166
✔️Some patterns of the relationship between structure and biological activity in the series of
2-aryl- and 2-heterylpyrrolidines have been revealed
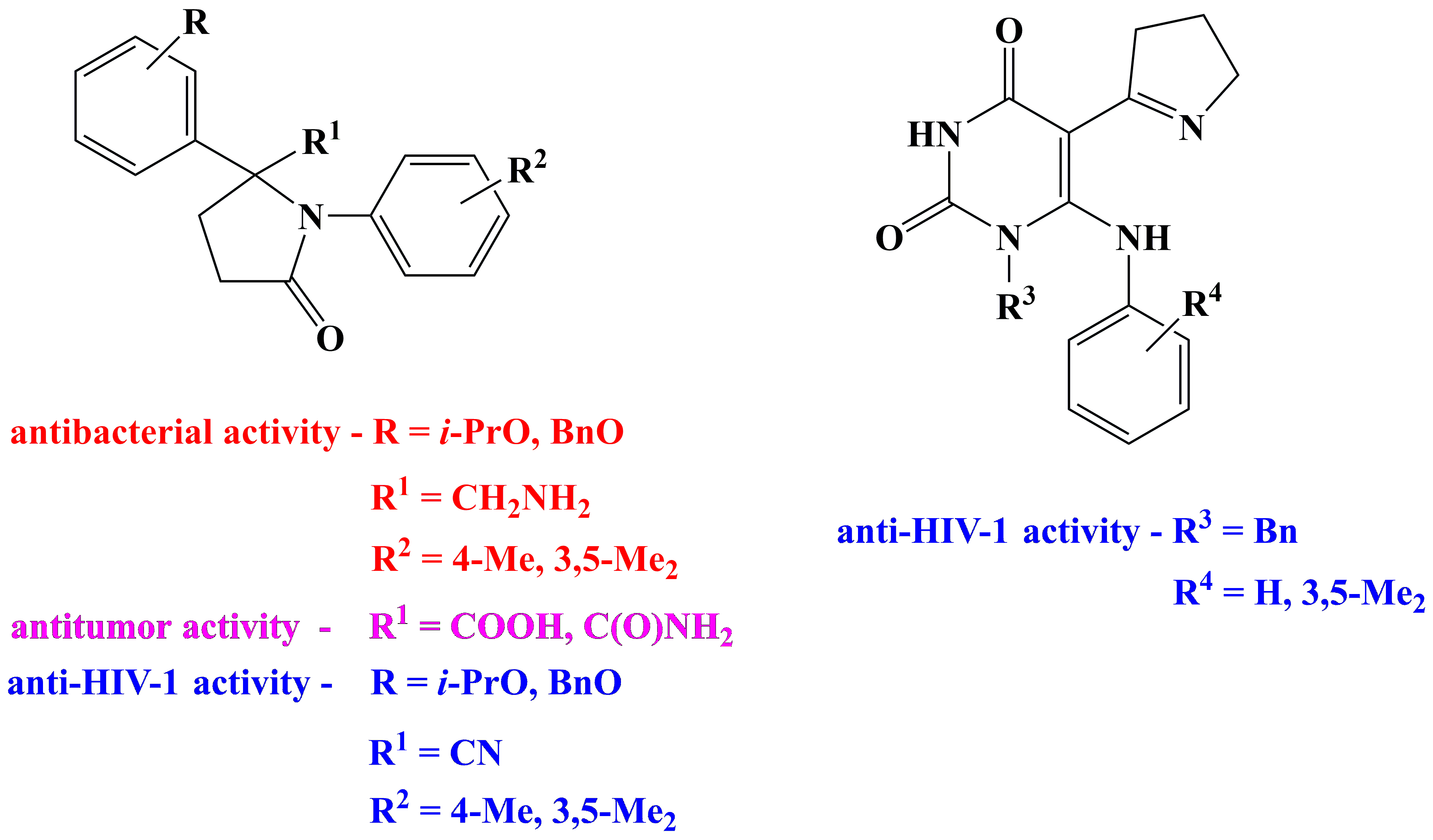
Pharm. Chem. J., 2012, v. 46, № 6, pp. 331-333. doi: 10.1007/s11094-012-0792-2
Chem. J. Armenia, 2016, v. 69, № 3, pp. 290-295
Chem. J. Armenia, 2017, v. 70, № 4, pp. 537-545
Med. Chem. Res., 2017, v. 26, № 1, pp. 101-108. doi: 10.1007/s00044-016-1731-7
Mol. Divers., 2021, v. 25, № 4, 2045-2052. doi: 10.1007/s11030-020-10095-1
✔️Various derivatives of 1,3-diazaadamantanes have been synthesized
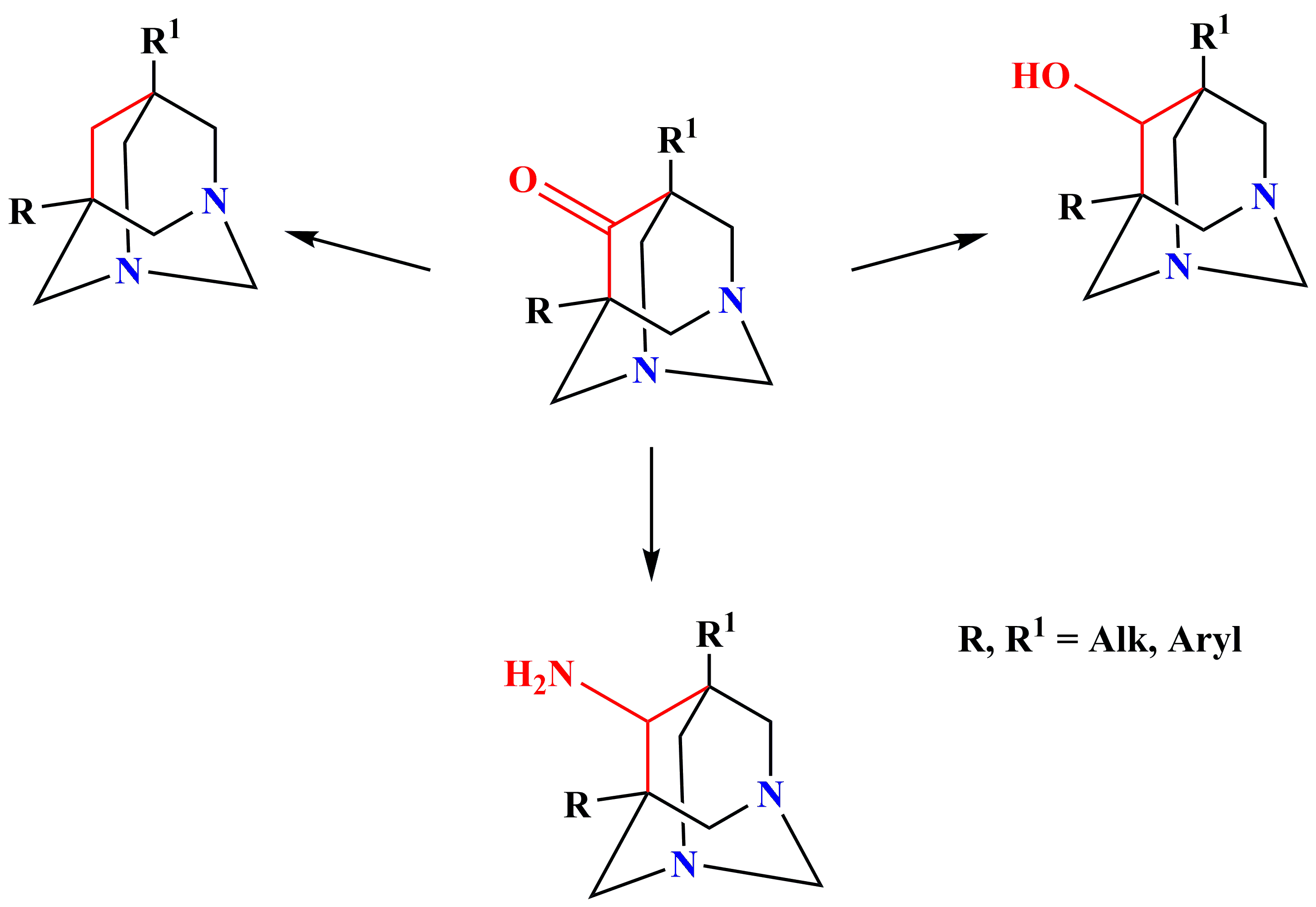
Chem. Het. Comp., 2012, v. 48, No. 11, pp. 1670-1675. doi: 10.1007/s10593-013-1191-7
✔️Based on 3,7-diazabicyclo[3.3.1]nonanes, a synthesis of new derivatives of 1,3-
diazaadamantanes was carried out
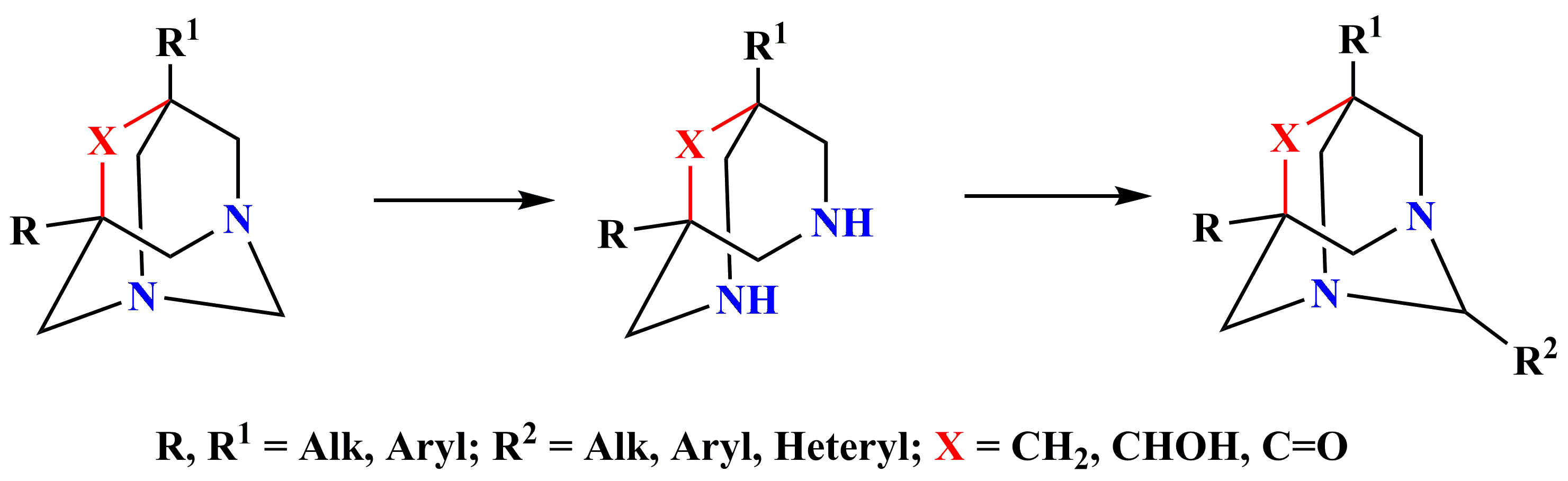
RA Patent № 2477 A (2011)
Mol. Biol., 2014, v. 48, No. 5, pp. 741-748. doi: 10.1134/S0026893314050100
Russ. J. Org. Chem., 2014, v. 50, No. 10, pp. 1480-1484. doi: 10.1134/S1070428014100133
Chem. Het. Comp., 2017, v. 53, No. 2, pp. 192-195. doi: 10.1007/s10593-017-2039-3
Phar. Chem. J., 2021, v. 54, No. 1, pp. 1205-1208. doi: 10.1007/s11094-021-02344-w
✔️Synthesis of 7-nitro-, 7-amino-1,3,5-triazaadamantanes and corresponding Schiff bases was
carried out
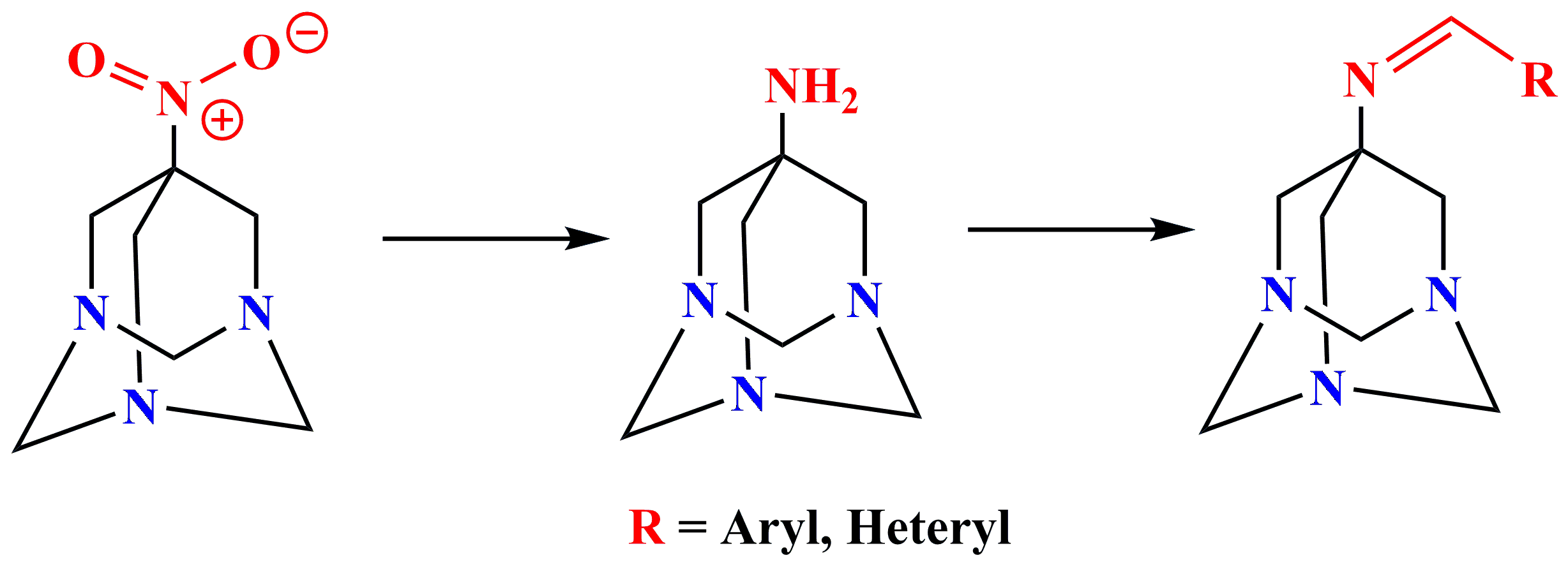
Pharm. Chem. J., 2018, v. 52, No. 5, pp. 419-423. doi: 10.1007/s11094-018-1834-1
The best works of recent years
Pharm. Chem. J., 2021, v. 54, No. 1, pp. 1205-1208. doi: 10.1007/s11094-021-02344-w
Mol. Divers., 2021, v. 25, No. 4, pp. 2045-2052. doi: 10.1007/s11030-020-10095-1
Pharm. Chem. J., 2023, v. 56, No. 11, pp. 1443-1450. doi: 10.1007/s11094-023-02810-7
FEBS Journal, 2023, 290 (23), pp. 5566–5580. doi: 10.1111/febs.16943
Pharm. Chem. J., 2024, v. 58, No. 5, pp. 730–737. doi: 10.1007/s11094-024-03200-3
Russ. J. Gen. Chem., 2024, v. 94, No. 5, pp. 1031–1039. doi: 10.1134/S1070363224050037
Russ. J. Gen. Chem., 2024, v. 94, No. 11, pp. 2809-2823. doi: 10.1134/S107036322411001X
Russ. J. Org. Chem., 2024, v. 60, No. 12, pp. 2393-2400. doi: 10.1134/S1070428024120121
BioTech, 2024, v. 13, No. 40, pp. 1-16. doi: 10.3390/biotech13040040
J. Struc. Chem., 2025, v. 66, No. 2, pp. 338-345. doi: 10.1134/S0022476625020118